Spectroscopy Lab instructions
Spectroscopy
Objective:To learn about the structure of an atom from the light it gives off, and to identify
elements by their spectra.
Background:The term spectroscopy means to learn about certain qualities of distant objects by the light they give off. These qualities can be the object's chemical makeup, its temperature, the speed it rotates or the speed it approaches or recedes.
Spectroscopy was advanced by two chemists in the mid-1800s: Robert Bunsen and Gustav Kirchhoff. They, primarily Gus, discovered that heated objects give off light in three very distinct ways:
If the object is hot and dense, like the core of a star or the filament of a lightbulb, the spectra is continuous.
If the object is hot and rarified (or sparse or low density), like a gas, the spectra is discrete. This is also known as an emission spectra.
If the object is cool and rarefied, it doesn't emit light: it absorbs discrete spectra. This is called an absorption spectra.
(The missing alternative, cool and dense, doesn't allow light to pass through it,
so we can't observe a spectrum.)
What's a spectra? (it's the plural of spectrum :) ) It's a luminous fingerprint, unique to an element. It takes the form of many-hued light. Continuous spectra are like a rainbow:

A discrete spectrum, also known as an emission spectrum, are like a series of bright lines against a black background:

Absorption spectra are like a rainbow with thin black lines in it:

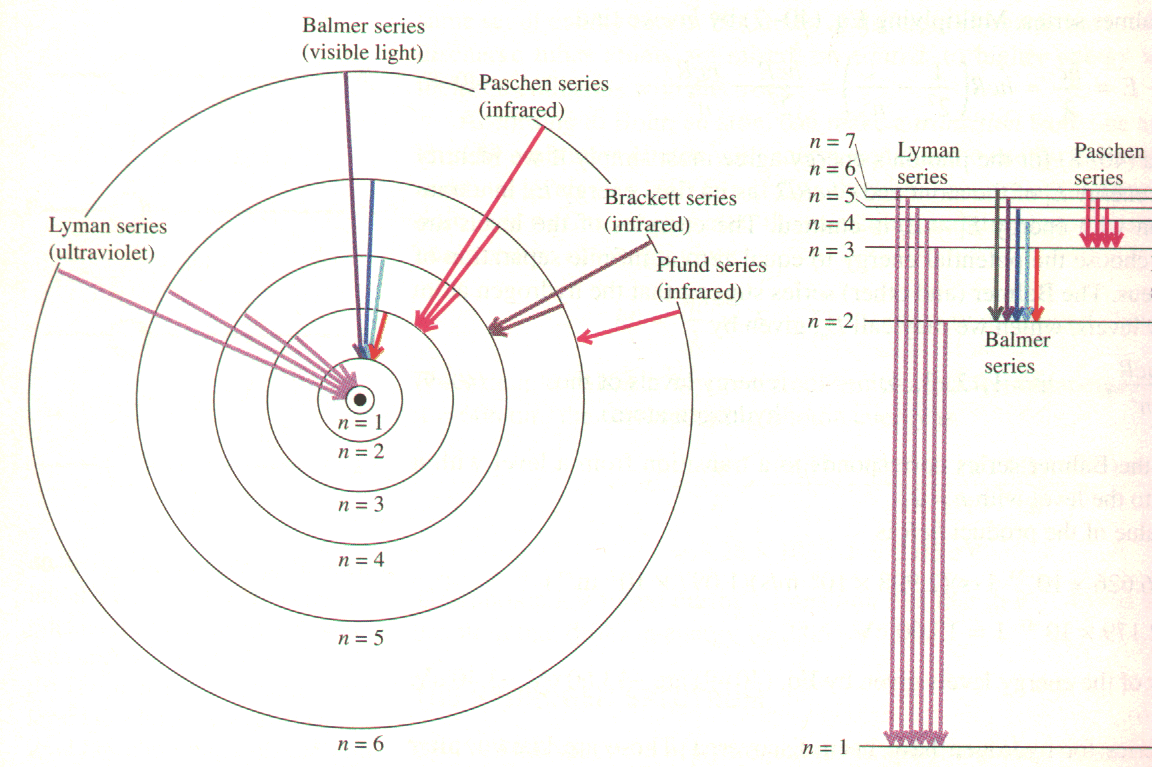
How can this be a fingerprint? Because of the process involved in making the spectra. Take, for example, discrete spectra. An atom of hydrogen has a single electron orbiting the nucleus of a single proton, making the model (called the Bohr model, after Danish physicist Neils Bohr) very simple. The electron, because of something called quantum mechanics, can only orbit certain distances from the atom. This is because the electron behaves like a wave instead of a particle when it is in the atom. As we saw in the light lab, waves can have constructive or destructive interference. The allowed distances for the electron correspond to where it has constructive interference. Destructive interference would mean the electron ceases to exist, and that is not allowed. These distances correspond to energy levels, denoted with the letter n where n = 1, 2, 3, 4.... A level n = 1 is the lowest state, called the ground state. Most of the time the electron is in the ground state, but if a certain amount, and just the right amount, of energy impinges on the atom, the electron can jump to a higher level. Check out this picture:
An animation may provide better insight into what is happening. Although there is no sound with it, here is a good animated explanation of atomic absorption and emission of light.
What it shows is that the electron can jump from n = 1 to n = 2, 3, 4, 5, 6 or beyond. It then orbits from a short time farther away from the proton than usual. But eventually it drops back down. In order to do this it gives up the energy it absorbed in the form of a quantity of light called a photon. The bigger the drop, the more energy is released, and the shorter the wavelength of the photon. In visible light, long wavelengths are red and short wavelengths are violet. Red light has lower energy photons than violet. You can think of this as red photons not having as strong of a punch as violet photons do.
But wait, there's more! See how sometimes the temporary "landing zone" is not n = 1? The temporary landing zone denotes a series. When you do the calculations, if n = 1 as an endpoint, the energy changes make photons with too much energy to see -- the photons are in the ultraviolet part of the electromagnetic spectrum. If the landing zone if n = 3, 4, 5... the energy changes make photons with too little energy to see -- these photons are infrared photons. So all we can see is when the landing zone if n = 2. This is called the Balmer Series.
So, each jump down to n = 2 generates a color of light unique to hydrogen. Voilà! A fingerprint. Sodium has its own energy levels, helium its own, etc. We can identify a substance at a distance by the colors of light it emits. We saw continuous spectra during the Light lab, and we'll use absorption spectra when we classify types of stars.
Trouble is, the different colors are mushed together. We need something to separate the colors. Two simple devices will do this: a prism and a grating. We'll use the latter. A grating is a piece of film with lines etched on it very close together, so close than one inch might have 15,000 lines! Because of quality of light that causes it to bend around corner, called diffraction, different colors will bend at different angles depending on their wavelength. AND this wavelength depends on the energy level drop, which can indicate the temperature of the gas! So spectra can tell us a lot.
You can read more about diffraction from the Hyperphysics site at Georgia State University. It's a little technical, but a good resource (ask me if you want an explanation).
Assignment: You will be recording the color/wavelength of various emission spectra. Best to make a chart for organizational purposes. How about this: one column for the colors, one next to it with the approximate wavelengths, and a third for the suspected identity. First, we'll practice with hydrogen using the RSpec Explorer software together as a class.
- Use the worksheet for this lab to sketch the spectrum for hydrogen; assign the wavelengths to their proper values on the scale.
The red line in the hydrogen spectrum is known as the H-alpha line. It should appear at 656 nm. This wavelength is used to study the sun's photosphere and chromosphere, as well as to identify where clouds of hydrogen (called nebulae) exist in space.
Method: I will set up various glowing tubes around the perimeter of the room. Aim the spectrometer at the tube and look off to the left (inside the spectrometer). You'll see emission lines unique to the element that is glowing. Also, there is a scale: 400 on the right to 700 on the left. These represent 400 to 700 nanometers, that is, 10-9 meters. Do your best to associate a number with each color. Focus on the lines that stand out the most.
Each tube contains one gas, HOWEVER, some corruption can occur, meaning that air might leak in. This will add lines that shouldn't be there. When you look up the comparison spectra on RSpec Explorer, match as many lines as you can. If there are wavelengths which don't fit, but seem to appear in every element, it's probably some kind of leakage.
Calculations: Actually, no calculations, only identifications. Use RSpec Explorer and the recorded videos of various gases to identify which element is in which tube. You already did hydrogen above - we didn't put hydrogen out here again, so make another guess! You can get the comparison spectra from the Dropbox that is present on the Desktop of the laptops. Go into the A105L folder and look for the recorded spectra of various elements. Grab the movie files for the elements you want to compare and drag them to the Desktop so you can look at them with RSpec Explorer.
Turn in your chart with the suspected identities of the glowing tubes. Each individual must turn in a chart with their guesses.

Stay Connected